Midline Thalamic Nuclei Display Elevated
Resting-State cFos Expression; Primary Sensory and Motor Nuclei Display
None:�
An Immediate-Early-Gene Functional Mapping
Study in the Macaque Thalamus��
S. Mikula*, S.H.C. Hendry�����
Dept. of Neuroscience, Johns Hopkins
University
Introduction
Expression
of the immediate-early-gene product, cFos, is an indicator of neuronal activity
that has been employed in multiple studies. In this study, the distribution of
neurons in the macaque thalamus immunostained for cFos following a period of
minimal sensory stimulation was determined. By allowing primates unrestrained
activity in the home cages for several hours, we attempted to produce
conditions characteristic of baseline stimulation for which immunostaining
under well-controlled conditions could be compared.
���� Our results show that the midline nuclei
express the highest levels of cFos, and that primary sensory and motor nuclei
do not express significant levels of cFos, in the macaque thalamus during the
resting state.
Abbreviations:� AD
anterodorsal n.; Al alaris n.; AM anteromedial n.; AV anteroventral n.; Cdc
densocellular central n.; Cif centroinferior n.; Cim centrointeromedial n.; Cl
centrolateral n.; Clc latocellular central n.; Cn.Md centre median n.; Cs
centrosuperior n.; Csl centrosuperolateral n.; GLd dorsal lateral geniculate;
GM medial geniculate; Hl lateral habenula; Hm medial habenula; LD laterodorsal
n.; Li limitans n.; LP lateroposterior n.; MD mediodorsal n.; Pa
paraventricular n.; Pcn paracentral n.; Pf parafascicular n.; Pt parataenial
n.; Pul pulvinar; R reticular n.; Re reuniens n.; Ro rhomboid n.; Ru ruber n.;
SG suprageniculate; VA ventral anterior n.; VL ventrolateral n. ; VPI
venteroposteroinferior n. ; VPL venteroposterolateral n.; VPM
venteroposteromedial n.; X area X; Zic zona incerta
Methods
Behavioral
Protocol:
Three
monkeys were kept in their homecages under minimal stimulation conditions
for eight consecutive hours prior to perfusion.�
By minimal stimulation conditions, we mean that the monkeys were allowed
unrestrained activity in their homecages but were not presented with any
additional stimuli that might otherwise bias our results; hence the denoting of
this behavioral protocol as a �resting state�.
Immunocytochemistry:
Monkeys
were perfused with 4% paraformaldehyde, and their brains subsequently removed,
blocked, sunk in 30% sucrose, frozen by immersion in powdered dry ice, and cut
at 16 microns on a sliding microtome.��
Sections were processed immunocytochemically for cFos (Santa Cruz
Biotech.), SMI-32, parvalbumin, calbindin, and calretinin, and histochemically
for acetylcholinesterase, cytochrome oxidase, and nissl, and subsequently
mounted onto subbed slides.�
Tracings:
All sections
were digitally photographed with a high-resolution CCD camera.� Thalamic areal boundaries were determined
from sections stained for acetylcholinesterase, parvalbumin, and calbindin and
traced out in Illustrator (Adobe).��
Thalamic nuclei were labeled according to Olszewski, 1952.� The distributions of cFos
immunoreactive nuclei was plotted onto the thalamic tracings.
Quantitation:
Thalamic
neurons immunoreactive for cFos were quantitated using simple immunoreactive
cell counts involving a 0.5 micron wide counting window and pseudo-random
sampling.� Cell counts thus obtained were
subsequently classified according to which thalamic nuclei they were obtained
from.
�
Results

Figure
2. cFos
Distribution in Thalamus of HM13L.� Each
red triangle corresponds to a cFos-ir neuron.
Figure
3. cFos
Distributions in Thalami of HM196 and HM201. Clusters of densely packed cFos
immunopositive nuclei were observed in the paraventricular and limitans nuclei
in all sections containing these nuclei. The primary sensory nuclei comprised
of the geniculate bodies and ventrolateroposterior nucleus were unique in
containing among the lowest levels of cFos expression, with typically only one
or two immunopositive nuclei being observed in single coronal sections.
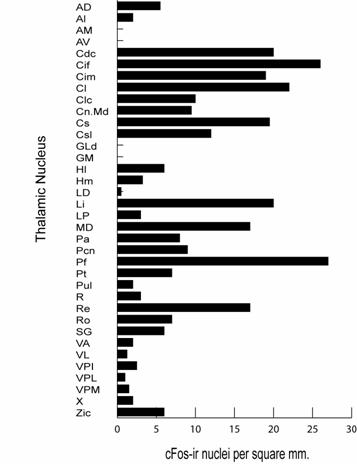
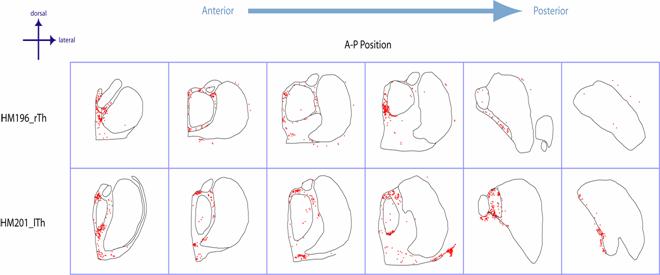
Figure
4. Summary of
cFos data for the three monkeys used in this study.� Note the marked elevation of cFos in midline
and intralaminar nuclei and the almost nonexistent levels in primary sensory
nuclei.
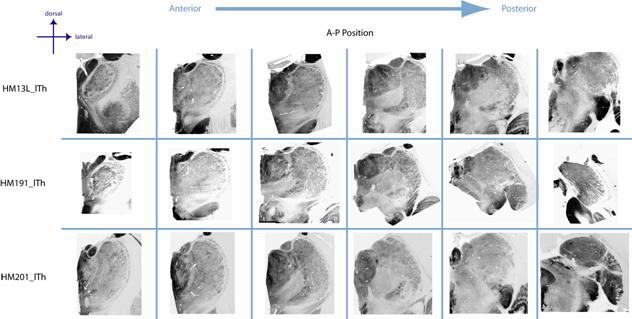
Figure
5. The pattern of thalamic cFos
immunostaining differed markedly from that seen for histochemical staining for
the mitochondrial enzyme cytochrome oxidase (CO). CO was very high in a large
number of nuclei, with the anterior nuclei and the geniculate bodies showing
the most intese CO activity. CO was lowest in a number of nuclei, which
included the intralaminar nuclei.
Conclusions
- When primates are allowed unrestrained activity in their homecages,
certain midline and intralaminar thalamic nuclei invariably express high levels
of cFos, whereas primary sensory nuclei do not express any.
- Our results suggest that certain midline and intralaminar nuclei
comprise part of a distributed neuronal system involved with maintaining a
�baseline� or �resting state�.
References
1. Morgan J.I. and Curran T. (1991).
Stimulus-transcription coupling in the nervous system:Involvement
of the inducible proto-oncogenes Fos and Jun. Annu. Rev. Neurosci. 14,
421�451.
2. Olszewski J. (1952). The Thalamus of the Macaca
Mulatta.� S. Karger. New York.
3. Searching for a baseline: functional imaging and the
resting human brain.
Nat Rev Neurosci. 2001 Oct;2(10):685-94. Review.
